Structure of the Periodic Table - 9/25-9/29
 |
| https://image.slidesharecdn.com/theperiodictable-110223090122-phpapp01/95/the-periodic-table-7-728.jpg?cb=1298451775 |
Summary:
This week, we have been learning about how the periodic table is arranged and the different patterns that are in the rows and columns. In the periodic table, the rows are called periods. Along the rows, the elements are organized by atomic number (the number of protons.) The rows also have the same energy level or the number of electrons. For example, in the first row, all of the elements' atoms have one energy level, and so on.The atomic mass is the number of neutrons plus the number of protons. The atomic mass can be a decimal, and it is ordered increasingly.
The columns in the periodic table are called groups or families. Each family has a name. For example, group one is composed of alkali metals which is the family with Lithium, Sodium, and more. Each group also has the same number of outer, or valence electrons. The elements in group one have one valence electron in their atom. Columns are also called families because they have similar chemical properties. Using the same example with alkali metals, all of the elements are very reactive.
Learning about an Element From the Periodic Table
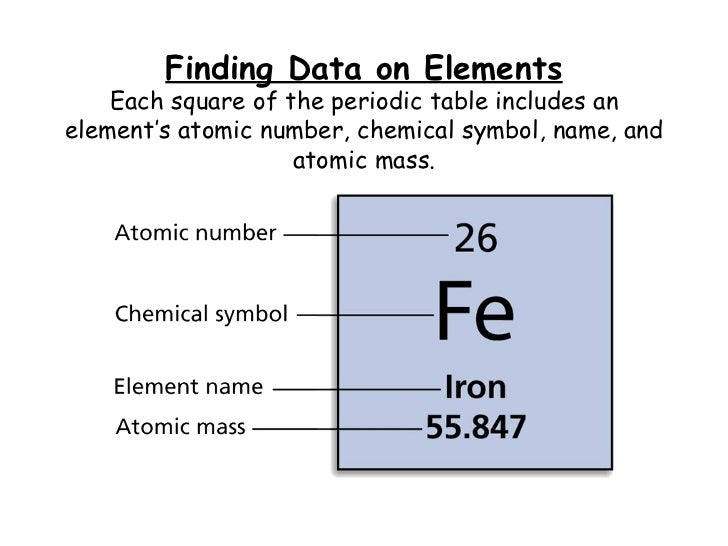
When looking at the periodic table, you see a bunch of squares, put together to form a giant "table." But what do those squares mean? On the periodic table, each element is represented by a single square. On each square, there are generally four pieces of information that you can gather by looking at the periodic table. The number on top is the atomic number. This is the number of protons that are in the atom. In the middle is a chemical symbol. This is never more than two letters, never has numbers, and only the first letter is capitalized. Under that is the element name. In this example, the element name is Iron. The last piece of information is the atomic mass, which is the number of protons plus the number of neutrons. From this, you can figure out the number of neutrons by finding the atomic mass and subtracting the atomic number.
As an example, in the image above, the element has 26 protons in the atom. This also means that it is the 26th element on the periodic table. The chemical symbol is Fe, representing the element name Iron. The atomic mass is 55.847 so we can figure out that there are 30 neutrons in the element. As you can see, there is important information that you can gather about the periodic table which can help you figure out its placement.
SP6: Constructing Explanations and Designing Solutions
This week, we constructed explanations about the periodic table and the structure so that we could create a periodic table with similar patterns and constructed with the same understanding. We also needed to construct explanations so that we knew how we would order all of our information in the same way. We also needed to know how to label each element on the periodic table based on atomic number, symbol, name and atomic mass so that we could design a solution to our periodic table.
XCC: Structure and Function
The structure of the periodic table originally created by Dmitri Mendeleev serves the function of being able to learn and see each of the elements visually and know which group they belong to and their different properties. The structure of the periodic table supports the function and placement of each element because it helps us understand more about the different elements and where they would fit with different patterns and more. There are some other periodic tables that serve the same function, but the structure is different. Understanding how the structure and function relate can help us understand more about the elements and how they react.
Multiplier
This week I was a creator. I did my best to answer all of the questions and contribute all of what I knew about the periodic table with my group. I said, "I will do it" when creating the periodic table, and I did my best to create a response and help my group understand when they asked me questions. I contributed to the project by doing my best effort. When creating the abstract, I did my best to communicate by findings clearly and give my best response.
Comments
Post a Comment